Scientist of the Day - August Kekulé
August Kekulé, a German chemist, was born Sep. 7, 1829. In the early 1860s, Kekulé was writing a textbook of organic chemistry, and he was trying to work out the structure for what had recently been designated the 'aromatic compounds', which included benzene and its derivatives (not all molecules that are aromatic to the nose are aromatic to the chemist, and vice versa). Benzene was a particular problem. Its chemical formula is C6H6, meaning one benzene molecule has 6 carbon atoms and 6 hydrogen atoms, and it is quite stable. The stability was the surprise, because normally 6 carbon atoms would have gobs of hydrogen atoms bonded to them. Methane, for example, is a hydrocarbon, like benzene, but its chemical formula is CH4, meaning its one carbon atom is able to bond to 4 hydrogen atoms. So why is the benzene molecule satisfied with only 1 hydrogen atom for each of its carbon atoms?
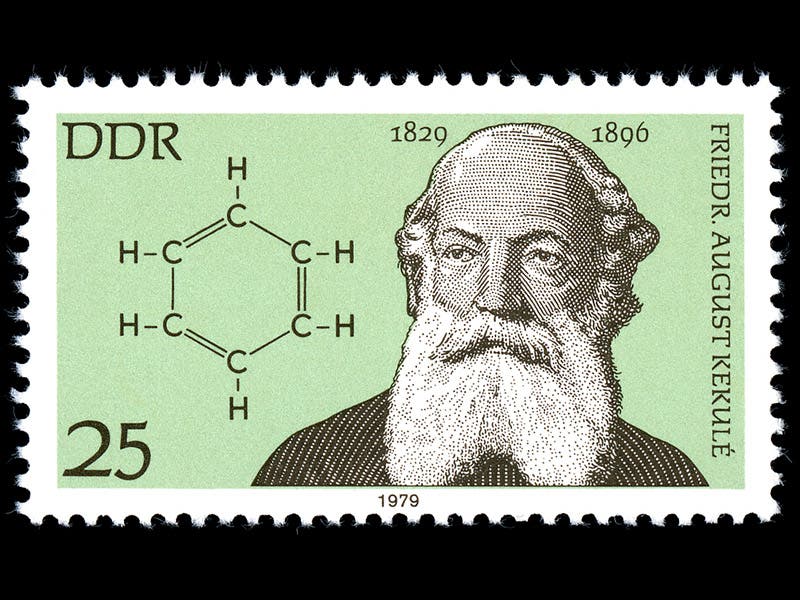
In 1862, Kekulé had an idea. Suppose the 6 carbon atoms form a circle, or a six-sided ring, so that the carbon atoms use most of their bonds on each other. The six empty bonds would then be occupied by the 6 hydrogen atoms, sticking out from the ring. The most attractive feature of the concept was that one could now see how benzene derivatives such as toluene and phenol might form, by replacing one of the hydrogen atoms with a different group of atoms, such as a methyl group (as in toluene) or a hydroxyl radical (as in phenol). A 1979 German postage stamp depicts both Kekulé and his benzene ring, with its alternating single and double bonds (first image).
Kekulé's proposed structure was brilliant, but its fame in the history of science stems from the way Kekulé came up with his brainstorm. At a meeting organized in 1890 to celebrate the 25th anniversary of the benzene announcement (his paper was published in 1865), Kekulé told the gathering that he got the idea of the ring from a daydream in which he saw a snake biting its tail. He literally dreamed up the benzene ring. We don't know if it actually happened this way, but most historians see no reason to doubt it, since the account came from Kekulé's own mouth and was delivered to a gathering of his peers. Others, however, are skeptical, and wonder if Kekulé were not just trying to buttress his claim for priority in the discovery of the benzene ring.
There is a handsome bronze statue of Kekulé in Bonn (second image); no tail-biting snake is in evidence, but there is a stool, which is perhaps intended to represent the site of the daydream. On the plinth below the statue (third image), there is a small relief, on which Nature, at the right, is handing a benzene ring, with its bent double-bonds, to Techne, essentially saying, “Here, make it work.” And if one looks in a wider view (fourth image) at the stone supports that hold up the walls on either side of the statue, you can make out their hexagonal core and 6 radiating spokes. There was nothing obvious about Hans Everding, the sculptor, if he designed the walls as well as the statue.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.