Scientist of the Day - Bertram Boltwood

Bertram Boltwood, an American radiochemist, was born July 27, 1870. In 1896, Boltwood was about to receive his PhD from Yale’s Sheffield Scientific School when the radioactivity of uranium was discovered. In 1903, while Boltwood was working privately as a chemist in New Haven, Ernest Rutherford in England announced that radioactive elements, when they emit radiation, actually transmute into other elements. He also revealed that radium-bearing minerals always contain helium, suggesting that helium is a byproduct of the transmutation process.
In 1904, Rutherford gave a talk at Yale that inspired Boltwood to investigate for himself the decay process of uranium, and when later that year, in St. Louis, Rutherford suggested that we might be able to calculate the age of the earth by comparing the amount of uranium in certain minerals with the amount of helium in those same rocks, Boltwood conceived his research program. He discovered that the ratio of uranium to radium in many rocks suggests that radium is itself a product of uranium decay. And then, in 1906, when Boltwood had just become an assistant professor at Yale, he discovered that uranium-bearing rocks always contain lead, suggesting that lead, which is not radioactive, is the final step in the cascade of uranium decay. Since the amount of lead in a uranium-bearing rock should increase over time, Boltwood saw the possibility of developing a way to date rocks.
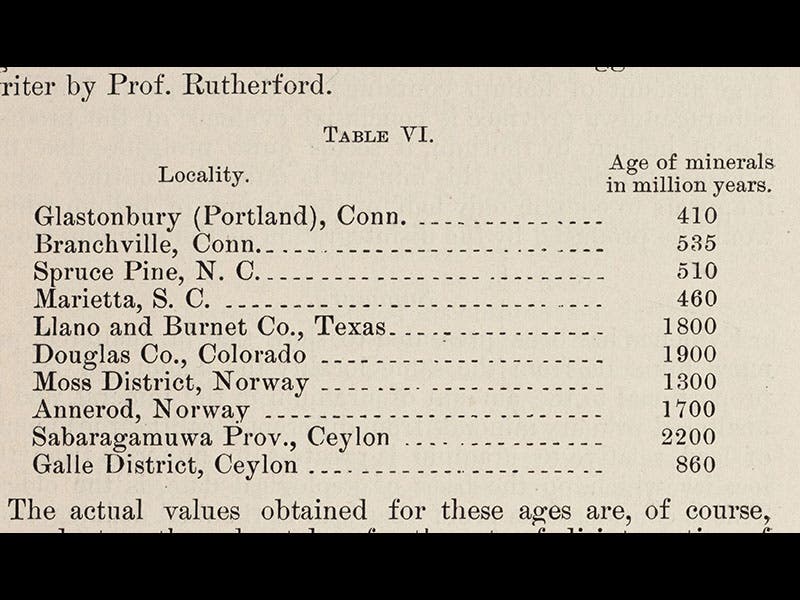
In 1907, Boltwood published a paper in the American Journal of Science that is a milestone in the history of radiometric dating (third image). He proposed that one could calculate the age of rocks by comparing the uranium and lead levels in those rocks. No one yet knew the half-life of uranium (at 4.5 billion years, it is difficult to measure), but they had a pretty good idea of the half-life of radium, and that is what Boltwood used in his calculations. He chose 20 different mineral samples, from all over the earth, whose lead and helium percentages he was able to measure, and for 10 of those he felt confident enough to calculate an age, which he provided in a table (fourth image). Most of the specimens yielded ages of 500 million years or so, which was many times larger than the commonly-accepted age of the Earth (100 million down to as low as 20 million years), and one of his specimens, a piece of thorianite from Sabaragamuwa Province in Ceylon (now Sri Lanka) yielded the staggering age of 2200 million (2.2 billion) years. When Boltwood’s proposed method of uranium/lead dating was confirmed by Arthur Holmes in 1911, the modern era of radiometric dating was off and running.
Boltwood apparently gave his rock samples to the mineral collection at the Yale Peabody Museum of Natural History, for they currently have three pieces of thorianite from Sri Lanka in their collection, with records indicating they came from Boltwood. You can see one of them above (first image), and access the record here. It is very likely that either this specimen (33330) or its companion (33328) was the one used by Boltwood to calculate an age of 2200 million years for an earth rock. We thank Stefan Nicolescu, Mineralogy Collections Manager at the Peabody Museum, for confirming on short notice that these specimens came from Boltwood.
There are surprisingly few photos of Boltwood about. The fifth image shows an older Boltwood at work in his lab at Yale. The second image should be much better known; taken in 1910, when Boltwood spent a year at the University of Manchester, it shows Boltwood (center), Rutherford (right), and at left, a young Otto Hahn, who would, 28 years later, be the first to split the atom.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.









