Scientist of the Day - Glenn Seaborg
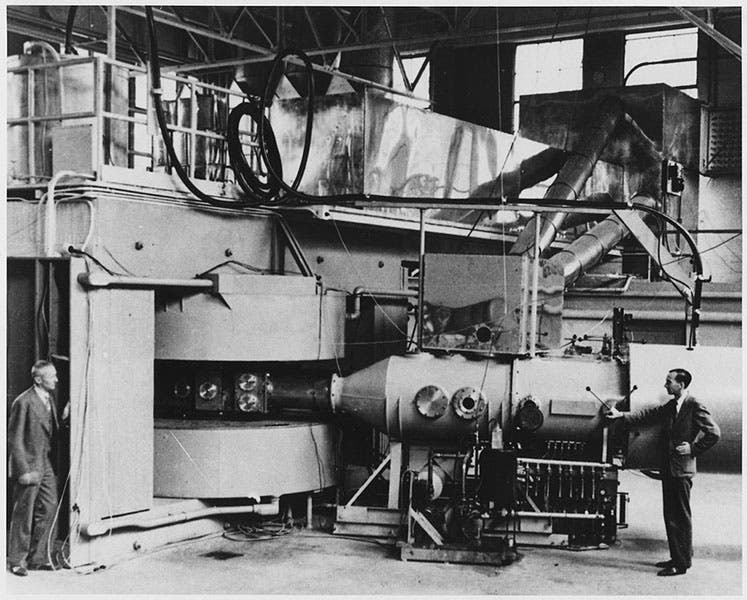
Glenn Seaborg, an American nuclear chemist, was born Apr. 19, 1912. In February of 1941, Seaborg led the team that discovered plutonium. They were working with the 60-inch cyclotron built by E. O. Lawrence at the University of California in Berkeley (second image, below). Less than a year earlier, Edwin McMillan, using the cyclotron, had discovered the first "trans-uranic" element, neptunium, which, with an atomic number of 93, has one more proton than uranium. Seaborg discovered that neptunium could emit nuclear electrons (by a process called beta-decay) to produce element 94. The hard part was separating the plutonium from the uranium and neptunium, which is where Seaborg’s training in chemistry came in. Since uranium carried the name of the 7th planet, and neptunium that of the 8th planet, Seaborg chose to call his new element plutonium, after the 9th planet. It is a good thing he did not foresee that Pluto would one day (2006) be deprived of its planetary status, or he might have worried that plutonium would be similarly demoted.
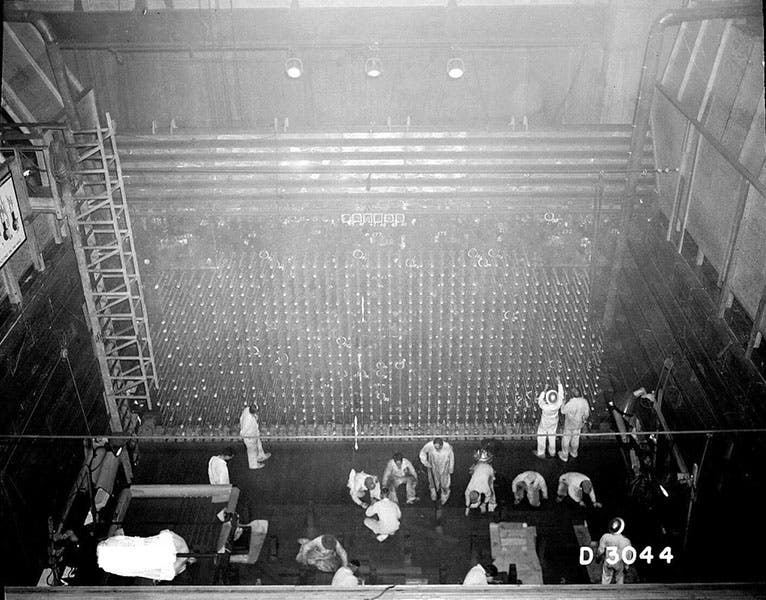
The discovery of plutonium proved to be of great importance for the Manhattan project, since it is fissionable, and so it could be used for a nuclear weapon. The Hanford B reactor in Washington state (third image, just above) by late 1944 was producing sizeable quantities of plutonium, and the Trinity "gadget" and the "Fat Man" bomb dropped on Nagasaki in 1945 were both plutonium bombs. In the fourth image, just below, we see 11.6 pounds of pure plutonium, enough to make one bomb, and formed into a ring rather than a ball for safety reasons. Seaborg had written a paper on his discovery, but he was not allowed to publish it until 1946, as a shroud of secrecy soon cloaked nuclear research in Great Britain and the United States. But he was awarded the Nobel Prize in Chemistry in 1951, which he shared with McMillan.
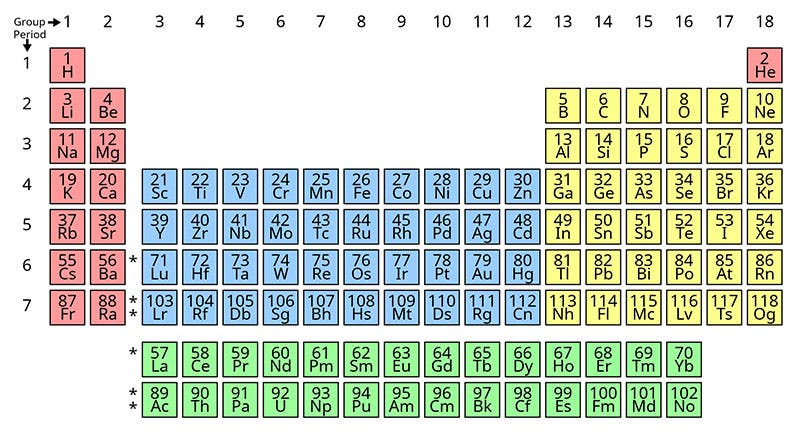
In the periodic table just above (fifth image), plutonium (Pu) is in the bottom row, just two to the right of Uranium (U), with neptunium (Np) in between. Most of the other elements to the right of plutonium (americium, curium, berkelium, etc.) were also discovered or co-discovered by Seaborg. Not shown is the unstable element no. 106, seaborgium, named after Seaborg, just before his death in 1999.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.