Scientist of the Day - Sadi Carnot



Sadi Carnot, a French engineer and a founder of thermodynamics, was born June 1, 1796. Carnot was educated at the famous Parisian military engineering school, the École Polytechnique, and after he retired from the army (at age 24!), he undertook to understand the physics behind the operation of steam engines, with a goal of improving their efficiency. Bear in mind, for the following discussion, that the prevailing theory of heat in Carnot’s day was the caloric theory, where heat was considered to be a fluid (“calorique”) that was transferred from body to body.
Carnot discovered that a steam engine works, not by consuming caloric, but by transferring heat from a warm reservoir to a cool one, using part of the heat to produce work. The greater the temperature difference between the two reservoirs, the more efficient the engine. This means that no engine can be 100% efficient, since some of the heat must be transferred to the cooler reservoir. Carnot also realized that if the heat reservoirs are at the same temperature, an engine will produce no work at all, even though the two reservoirs might contain a great deal of heat between them.
Carnot published his observations and conclusions in 1824 in Reflexions sur la puissance motrice du feu (Reflections on the motive power of fire). Since the book is now acknowledged as a milestone work in thermodynamics, it is curious that it had virtually no impact until another French engineer, Émile Clapeyron, spoke favorably about Carnot's work in 1834, and it wasn’t until the 1850s that Carnot’s discovery that not all energy is available for work was reworked by Rudolf Clausius and William Thomson (later Lord Kelvin) into the second law of thermodynamics. Carnot was unable to advance his own cause because he died in a cholera epidemic in 1832, at age 36. Since the Reflexions attracted almost no attention during his lifetime, it rapidly disappeared from the book market, and today it is very scarce for a milestone book. We have a handsome copy in the History of Science Collection (fifth image), made more special because it came from the library of the German physicist Ernst Mach (third image).
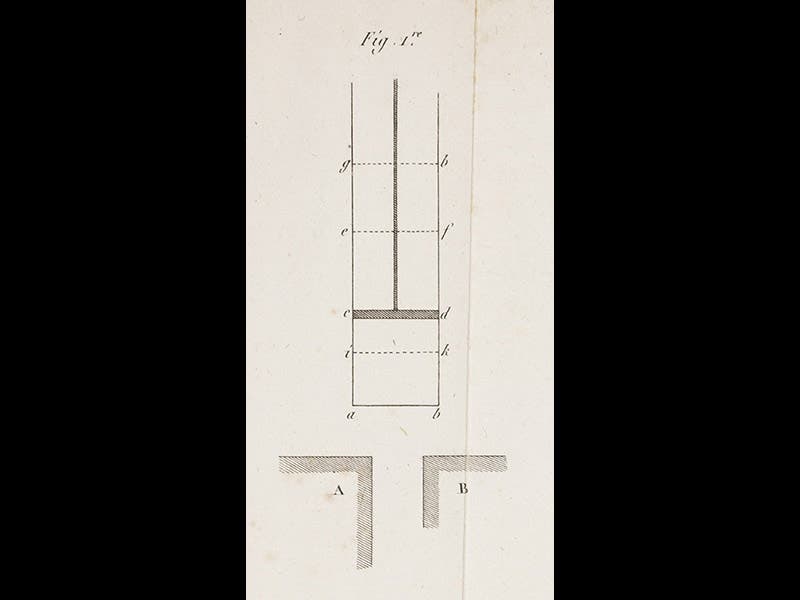
The one engraved plate from the Reflexions (fourth image) shows what we now call a Carnot engine; a detail of figure 1 (first image) shows a piston cd in a cylinder that will move up when placed in contact with the hot reservoir A and move back down when it abuts the cold reservoir B.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.







