Scientist of the Day - Willard Libby
Willard Libby, an American chemist, died Sep. 8, 1980, at the age of 71. Libby worked at Berkeley Radiation Labs before World War II, when the lab was interested in finding new isotopes of ordinary elements that might be useful for medical purposes, and one of the elements examined was carbon. It turned out that carbon has two unstable isotopes, carbon 13, and an extremely rare form, carbon 14. Carbon 14 differs from ordinary carbon 12 in having two extra neutrons. To everyone’s surprise, C14 decays very slowly (most medical isotopes decay extremely rapidly, which means one has to hotfoot it from the particle accelerator to the hospital before the isotope decays away). Still before the war, it was also discovered that, although humans can make C14 in the lab, nature also does so on her own, in the upper atmosphere, where cosmic rays turn ordinary nitrogen 14 into carbon 14 at a constant rate.
During the war, Libby joined the Manhattan Project, continuing to work on naturally occurring radioactive isotopes in our atmosphere. This did not prove useful for weapons research, but it occurred to Libby at some point that if C14 is always present in the atmosphere, then living creatures are going to take it in, and continue to renew it while they are alive, but after they die, the C14 in their bodies will slowly decrease. He thought therefore that it might be possible to develop a dating technique that utilized the decay rate of carbon 14.
Libby moved to the University of Chicago after the War. It had been determined that the half-life of C14 is about 5700 years, which means that if one measures the C14 level in something that had been dead for 5700 years, the C14 level should be half that of a now-living organism. The difficulty was in measuring the C14 levels, since it is present in such miniscule amounts, and the technique of measurement is what Libby worked on for 4 years. He was ultimately successful, and in 1949, Libby published two articles in the journal Science.
The first paper, published in March, with his PhD student Ernest Anderson and his post-doc James Arnold, described the Geiger counter set-up they had devised to screen out background radiation, and announced the results of a study to determine how much C14 is present in a variety of living things around the world, as well as a few preliminary attempts to measure C14 levels in objects no longer living. That is Anderson who is posing with Libby in the photo above.
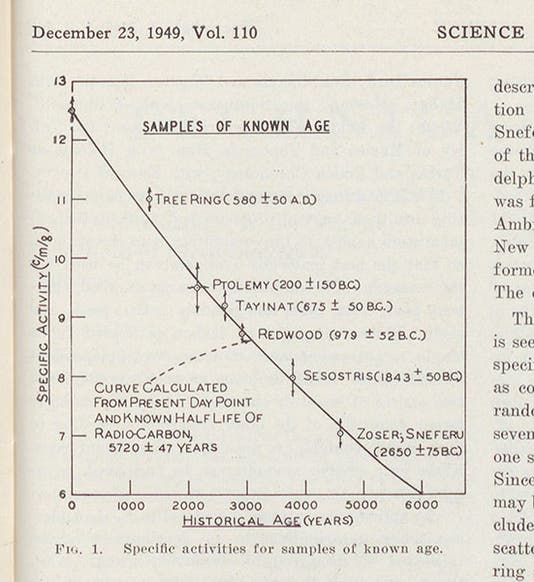
The second paper, published in later December of 1949, is the famous one. Here Libby and Arnold measured C14 levels and calculated ages for six wooden objects whose age was already known by other means, such as an ancient redwood, whose age could be determined by tree-ring counting, and a timber from the tomb of the pharaoh Zoser (found in the earliest Egyptian pyramid). Libby's dates were very close to the given dates, indicating that his method was reliable (he found Zoser's tomb to have been built in 2800 B.C.E. +/- 200 years; Egyptian archaeologists had settled on 2625 +/- 75 years). The graph in the paper, often reproduced since, told it all, as good graphs often do (first image). The usefulness of radiocarbon dating was immediately recognized; it would revolutionize archaeology in the succeeding decades, and it has since been used to date such disparate objects as the Chauvet cave paintings (32,000 B.C.E.) and the Shroud of Turin (1323 C.E. +/- 61 years, sorry). Libby received the Nobel Prize in Chemistry in 1960 for his discovery.
We once noted how few scientists were featured on the front page of Time Magazine during its lengthy heyday as the foremost news weekly in America. Libby was one of those few; he appeared on the cover of Time on Aug. 15, 1955, which was well before he received his Nobel Prize, so Time was actually doing some investigative reporting in this case (fifth image, above). When Time finally redeemed itself in scientists’ eyes by publishing an issue with no fewer than 15 scientists on its cover (the “Men of the Year” issue), on Jan. 2, 1961, Libby was again included, but this was just after his Nobel had been conferred, so no journalistic laurels are awarded for this occasion. Libby is just to the right of the atomic nucleus at the center of the cover (sixth image, below).
As a kind of footnote, Libby used a thick steel shield to surround his Geiger-counter setup in 1946-48 and screen out background radiation. Because all steel made after Aug. 15, 1945, was corrupted with fallout from atomic weapons, he used the steel from a pre-atomic-age battleship. Libby later gave the massive shield to the Atomic Energy Commisson (for which he was a commissioner, 1954-59), and it was acquired about 10 years ago by the Science History Institute. They apparently do not quite know what to do with it, but they did sponsor a nice video that you can view here, if you are partial to scientific artifacts, as I am. Thanks to Ben Gross for calling my attention to Dr. Libby’s shield.
William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.