Scientist of the Day - J. J. Thomson

Linda Hall Library
Linda Hall Library
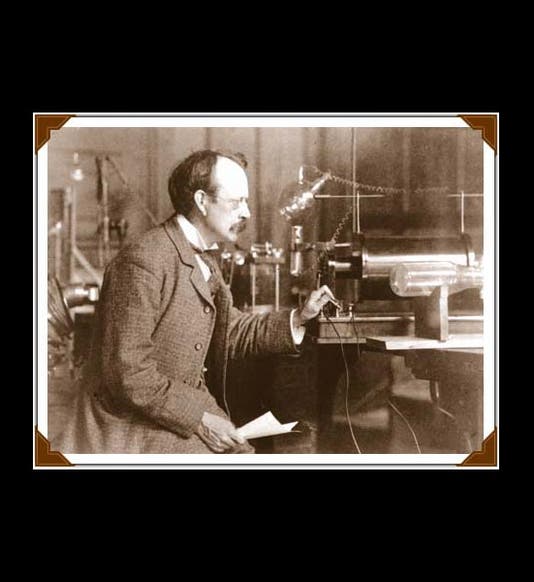
Joseph John Thomson, an English physicist usually known as J.J., was born Dec. 18, 1856 (first image). In 1884, Thomson, only 28 years old, was named the Cavendish Professor of Physics at Cambridge University. The Cavendish Lab had been founded just ten years earlier (second image). Its first director was James Clerk Maxwell, the great theorist of electromagnetism, so Thompson was stepping into some size triple-E shoes, and he managed to fill them quite respectably. In the 1890s, Thomson turned his attention to cathode rays. Cathode rays had been discovered in the 1870s by William Crookes, who invented something called a "Crookes tube." This long clear-glass tube was about the size and shape of a two-legged dachshund, with two electrodes inside. If one evacuated the tube, leaving just a little gas behind, and connected the electrodes to a battery, then the tube would glow, indicating that something was being emitted from the cathode (the negative electrode). A caricature of Crookes from Vanity Fair shows him holding one of his tubes (third image). We would call it today a cathode-ray tube.
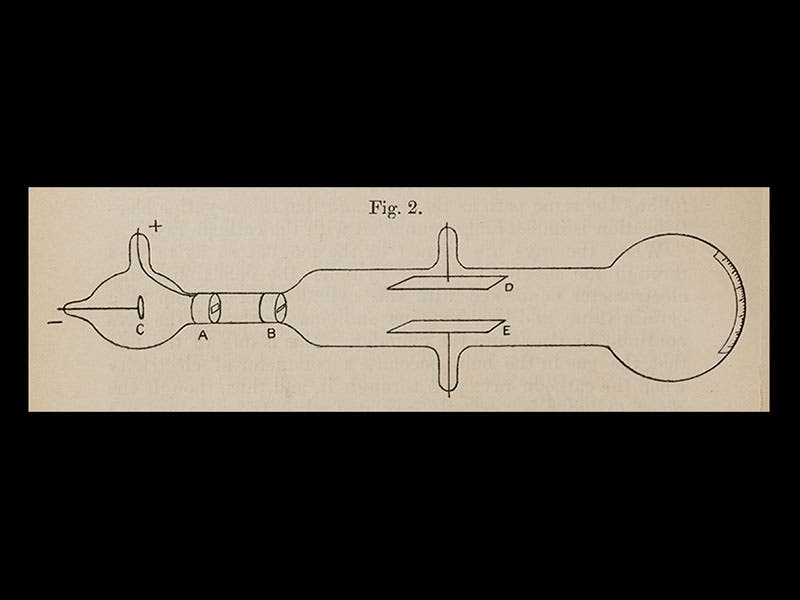
So Thomson was studying cathode rays and trying to determine their properties. He learned first of all that the rays could be deflected by a magnet, and because of the direction of the deflection, he concluded that the particles, if they were particles, had a negative charge. Then he determined how fast they were going, and when he compared that to the size of the deflection, he realized that the particles must have very little mass--they were in fact a thousand times less massive than a hydrogen atom, the smallest entity then known (in other words, a hydrogen ion, travelling at the same speed, would be deflected a thousand times less, and in the opposite direction, indicating it was positively charged and 1000 times heavier). Thomson called his particles "electrons", and he announced their discovery in 1897, in the London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. For the first time, the atom was found to have parts. We see above the first page of his paper of 1897 (fourth image), and a diagram from that paper of his experimental set-up (fifth image). The cathode-ray tube with which he discovered the electron is on display at the Cavendish Laboratory Museum (sixth image).
For his discovery of the electron and other work, Thomson received the Nobel Prize in Physics in 1906. This was the 6th year that the prize had been awarded. Thomson presided over the Cavendish Laboratory until his retirement in 1919, when he was succeeded by Ernest Rutherford. There is a plaque outside the old Cavendish Lab that commemorates Thomson’s discovery of the electron (seventh image).
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.