Scientist of the Day - John A.R. Newlands



John A. R. Newlands, a British chemist, died July 29, 1898, at age 60. In the early 1860s, Newlands took note of the repeating nature of the properties of the chemical elements, and he became one of the first to attempt to account for the phenomena with a periodic arrangement, some years before Mendeleev fashioned his periodic table in 1869. Newlands discovered that if he arranged the elements in the order of their atomic weights, then the eighth element has the same properties as the first, and the fifteenth shares those properties as well. He claimed that the elements obeyed what he called a "law of octaves", drawing an analogy to the musical scale, which repeats in the same pattern.
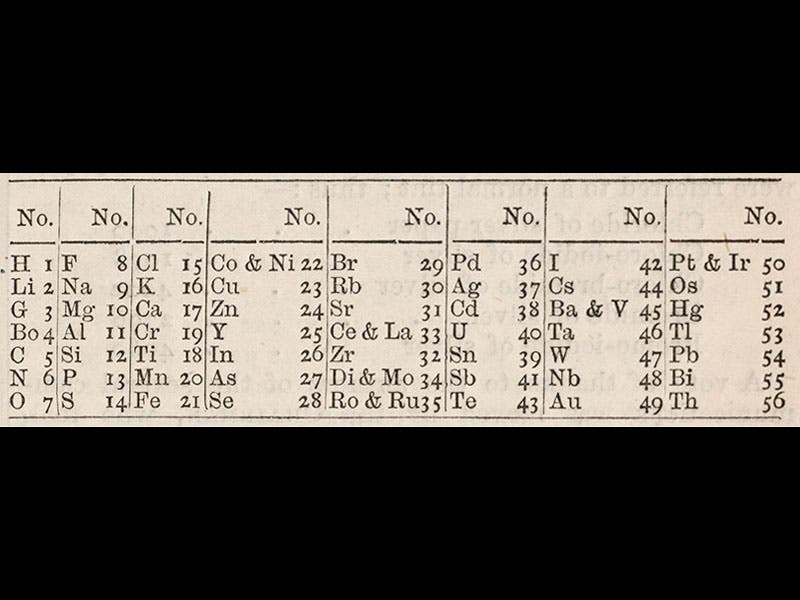
Newlands published a paper announcing his law of octaves in the trade journal Chemical News in 1865, including a graphical depiction of his periodic table (first image). A detail showing just the 1865 periodic table can be seen in the second image. Newlands then presented his arrangement to a meeting of the Chemical Society of London on Mar. 1, 1866. The Chemical Society would not publish his paper in their own journal, but Chemical News printed an account of the presentation on Mar. 9, 1866 (third image), and included Newlands' table (fourth image).
The Newlands periodic table is arranged differently than the later table of Mendeleev, with the elements going down in columns instead of across in rows, but Newlands did have fluorine and chlorine in the first row, sodium and potassium in the next, and magnesium and calcium in the third, thus successfully bringing together elements with similar properties. Newlands did not make many converts; one listener in the audience (says Chemical News) puckishly asked whether Newlands had tried ordering the elements alphabetically. It is not often noticed that the 1865 and 1866 tables differ slightly, especially in the ordering of the last column of elements.
After Mendeleev had published his arrangement and was subsequently praised for being the discoverer of the periodic table, Newlands lobbied hard for credit for devising the first periodic table, and he even republished all his Chemical News contributions in a little book, On the Discovery of The Periodic Law (1884), that we have in our Collections. But the Chemical Society (now the Royal Society of Chemistry) refused to take up his cause. It was not until 1998, on the centennial of his death, that a blue plaque was finally placed on his birthplace, proclaiming Newlands “discoverer of the Periodic Law for the chemical elements.”
There is apparently only one surviving portrait of Newlands, and perhaps only one print of that portrait, in the Edgar Smith collection at the University of Pennsylvania. It does not portray the young, 28-year-old Newlands who presented the first periodic table in 1865, but at least it shows our man. He does not look as beaten down as one might expect, after 30 years of disrespect.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.