Scientist of the Day - Stanislao Cannizzaro
Stanislao Cannizzaro, an Italian chemist, was born July 13, 1826. Cannizzaro was a very accomplished organic chemist, but he is best known for a single paper he published in 1858. Amedeo Avogadro had proposed, almost 50 years earlier, that the basic chemical unit is the molecule, not the atom. Avogadro had also hypothesized that equal volumes of gases under identical conditions contain equal numbers of molecules, allowing one to calculate atomic weights. However, Avogadro's hypothesis had not still not been accepted by most chemists, and some of the most influential, such as Jons Berzelius, had rejected it entirely. In his 1858 paper, called "Sunto de un corso di filosofia chimica" (“Sketch of a course in chemical philosophy”), Cannizzaro attempted to resurrect Avogadro and demonstrate how his hypothesis would allow chemists to measure atomic weights unambiguously. Determining atomic weights at the time was the subject of great controversy, primarily because atoms and molecules were often treated interchangeably.
Cannizzaro's paper, published in volume 7 of the Pisan journal Il Nuovo Cimento, did not initially attract many readers, but it was republished as a pamphlet in 1859. In 1860, the first international congress of chemists met at Karlsruhe in southern Germany, with the sole purpose or sorting out the basic units of chemical interaction and the proper methods for determining unequivocal atomic weights. Cannizzaro spoke at the Congress, recapitulating his paper and defending Avogadro, and Cannizzaro was apparently quite persuasive. At the end of the conference, copies of the reprint of Cannizzaro's "Sunto" were distributed to participants, and many seem to have read it on the way home, thereby becoming converts to Avogadro's molecular theory and his hypothesis. Lothar Meyer of Germany was one of those; he immediately began to recalculate the atomic weights of the elements and saw at once the possibilities of a periodic arrangement of the elements. Dimitrii Mendeleev was a recent graduate and just getting started, but he attended and later recalled Cannizzaro's skillful defense of Avogadro. The Karlsruhe Congress was perhaps the seminal chemical event of the decade (excepting Mendeleev's publication of his first periodic table in 1869), and the dissemination of Cannizzaro's paper was the highlight of that conference. Cannizzaro's place in the history of chemistry was established then and there.
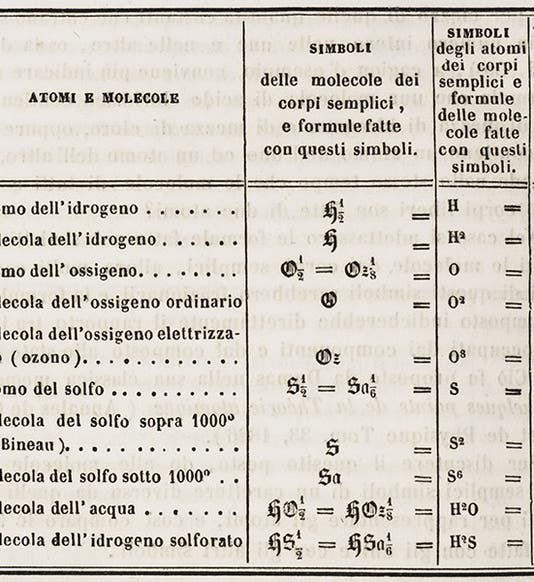
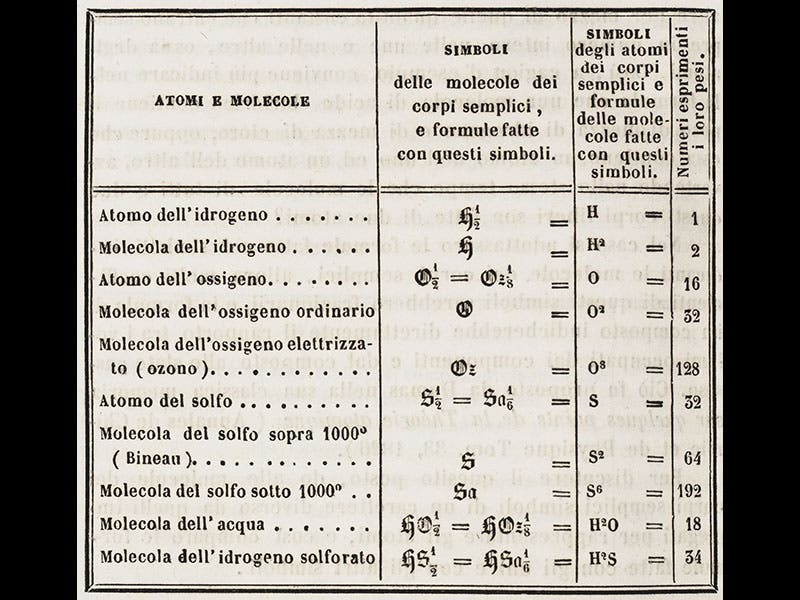
We have a copy of Cannizzaro's original paper in our serials collection; we see above the title page of the journal, the first page of Cannizzaro's "Sunto", and a table from the paper showing molecular and atomic weights. Cannizzaro's paper was translated into English in 1910 and published as part of the Alembic Club reprint series; we have that as well. But we do not have the separate printing of 1859 that was distributed at Karlsruhe; we would hope to acquire that one day.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.