Scientist of the Day - Francis Aston
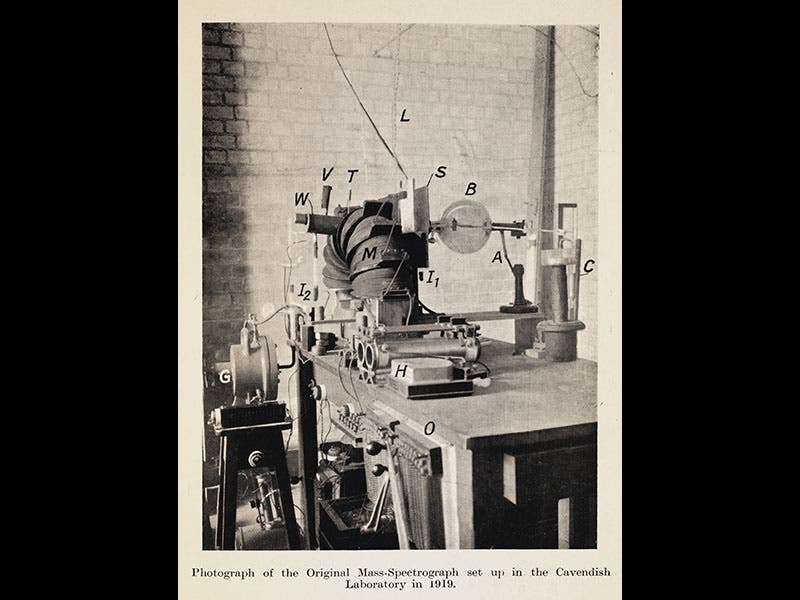
Francis William Aston, an English chemist turned physicist, was born Sep. 1, 1877. Aston was at the Cavendish Lab in Cambridge in 1913 when his boss, J. J. Thomson, discovered that the element neon seemed to have two different atomic weights, one about 20 times as heavy as hydrogen, and the other about 22 times heavier. These came to be called isotopes, and it was suspected that other elements had different isotopes as well, but there was no easy way to separate them and determine their atomic weights. In 1919, Aston invented the mass spectrograph (second image). This instrument takes ions boiled off a cathode and sends them through a magnetic field, which bends the ion stream into a loop. If several isotopes are present, then the heavier isotope will be curved less, and the ion stream will be divided into two (or more) parts, which will then produce two (or more) spots on a photographic plate, revealing the existence and, if done precisely, the atomic weights of the isotopes.
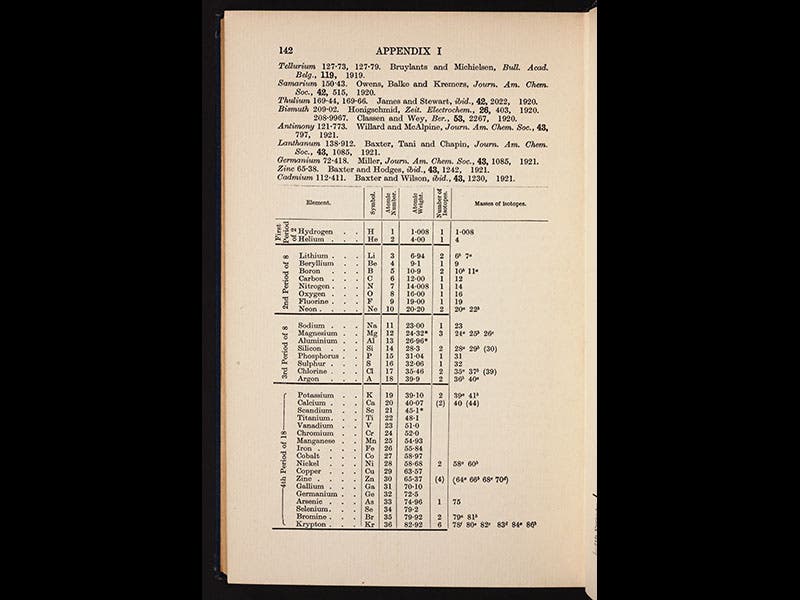
Aston discovered more than 50 isotopes in his first 6 years with his invention, and 212 in his entire career. The discovery of isotopes solved one of the big mysteries of chemistry--why many elements have fractional atomic weights. If elements are built up of the constituents of hydrogen atoms, as was suspected, then the weights of all elements should be even multiples of the weight of hydrogen, but they were not. Aston explained why. Neon has an atomic weight of 20.2 because it is a mixture of Neon 20 and Neon 22, in the ratio of about 9 to 1.
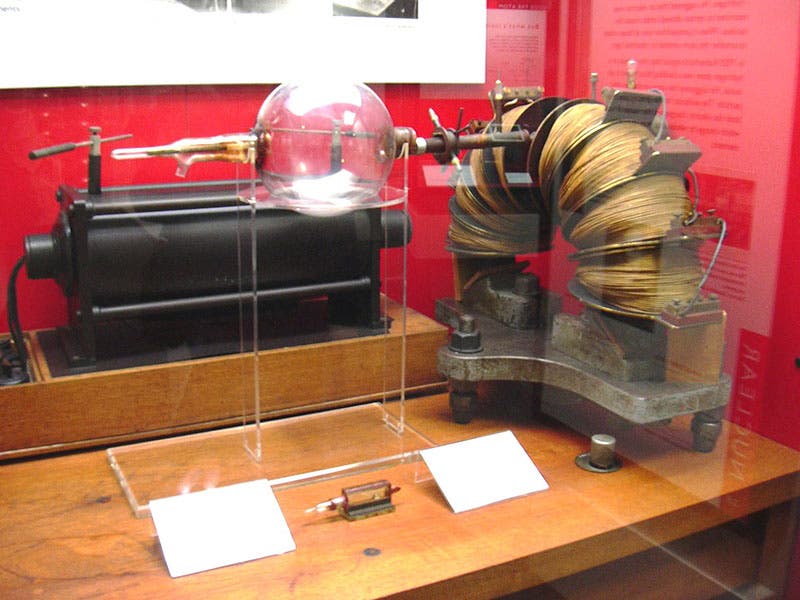
Aston's discoveries were so dramatic, and so significant, that he was awarded the Nobel Prize in Chemistry in 1922, the very year his book Isotopes was published. The second image above is from our copy of this book, as is the third, which shows the first part of a table of the elements. The right-most column presents the new isotopes, every one of them discovered by Aston. The other images depict: Aston at work in his lab with a later version of his mass spectrograph (first image); J.J. Thomson, Ernest Rutherford, and Aston (fourth image); a modern photo of the Old Cavendish Lab, where Aston worked (fifth image); and the original mass spectrograph of 1919, currently on display in the Science Museum, London (sixth image).
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.