Scientist of the Day - Friedrich Mohs
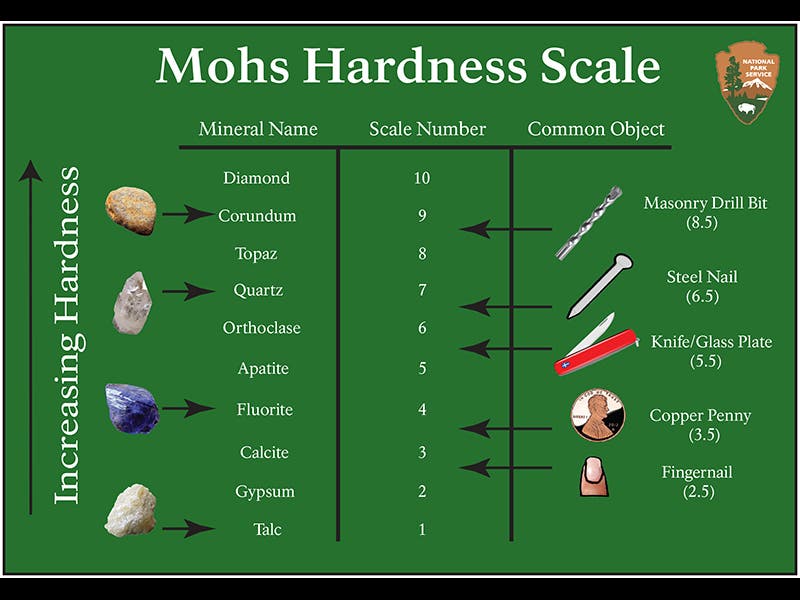
Friedrich Mohs, a German mineralogist, was born Jan. 29, 1773. In 1812, Mohs became professor and curator at the Joanneum Museum in Graz, Austria, where he worked out his famous hardness scale for minerals. The scale is defined by ten minerals, numbered from 1 to 10, each of which will scratch the minerals below it. So talc is hardness 1 and won't scratch anything. Calcite (3) will scratch gypsum (2), but is scratched by quartz (7). Corundum (9) will scratch nearly anything except diamond (10). We see above a modern version of the Mohs scale (second image)
The Mohs scale is unusual in that the numbers don't mean anything except rank. Calcite (3) is three times harder than gypsum (2), but corundum (9) is twice as hard as topaz (8), and diamond (10) is four times as hard as corundum (9). This makes the Mohs scale quite different from everyone's other favorite 1-to-10 scale, the Richter earthquake scale. On the Richter scale, a magnitude 6 quake is precisely 10 times more energetic than a magnitude 5 quake, and a magnitude 7 quake is 10 times greater than a 6. You can actually calculate the energy of an earthquake from its Richter number. The Mohs scale, on the other hand, does not really tells us anything except what mineral scratches what.
Mohs went on to succeed the great mineralogist Abraham Werner as professor at Freiberg in 1817, and then became professor of mineralogy in Vienna in 1826. He is remembered in Graz by a bust in what is now the Universalmuseum Joanneum (first image). But his greatest monument has to be a memorial plaque erected by the citizens of Vienna to honor their adopted mineralogical son. It depicts Mohs and his scale, with each of the ten Mohs minerals shown in colorful relief (third image). One would suppose that the diamonds at the top are not real.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.





![Andromeda and Perseus, constellations figured by James Thornhill, with star positions determined by John Flamsteed, in Atlas coelestis, plate [15], 1729 (Linda Hall Library)](https://assets-us-01.kc-usercontent.com:443/9dd25524-761a-000d-d79f-86a5086d4774/1b30cfec-5be6-4297-a7fb-97255ba992e5/thornhill1.jpg?w=210&h=210&auto=format&fit=crop)


