Scientist of the Day - Gustavus Hinrichs
Gustavus Detlef Hinrichs (first image), a Danish-American physical chemist and polymath, was born on December 2, 1836. Hinrichs grew up in the Duchy of Holstein, a German-speaking region that was once part of the Holy Roman Empire but had been under Danish rule since the 15th century. As a boy, he watched as war broke out between Denmark and Prussia over the political future of Holstein and the neighboring territory of Schleswig. By the time he graduated from the University of Copenhagen in 1860, diplomatic tensions between the two countries were once again on the rise, which likely inspired his decision to immigrate to the United States the following year. After crossing the Atlantic, Hinrichs worked as a high school teacher before successfully applying for a professorship at the University of Iowa.
Hinrichs originally served as head of the University’s department of Modern Languages, but in light of his scientific training, he later received an appointment as Professor of Natural Philosophy, Chemistry, and Modern Languages. Eventually, another faculty member took over his language classes, allowing Hinrichs to focus on reforming the school’s science curriculum. He wrote his own chemistry and physics textbooks and transformed the University of Iowa into one of the nation’s leading destinations for hands-on laboratory instruction.
In addition to being a popular lecturer, Hinrichs was a prolific author. Between 1856 and 1913, he published twenty-five books and hundreds of articles. Among historians of science, Hinrichs is best known as one of several contributors to the development of the periodic table. In 1859, German chemists Robert Bunsen and Gustav Kirchhoff demonstrated that chemical elements emitted different colors of light when heated. Bunsen and Kirchhoff separated each spectrum using a prism and carefully measured the distances between the various colored lines. Hinrichs became convinced that these measurements could provide valuable information about the atoms that made up each element. “As soon as I heard of the great discovery of Kirchhoff and Bunsen,” he wrote in 1864, “I felt sure that the dark lines of the elements would prove to be distributed according to simple laws, and that these laws might lead us to a knowledge of the relative dimensions of the atoms.”
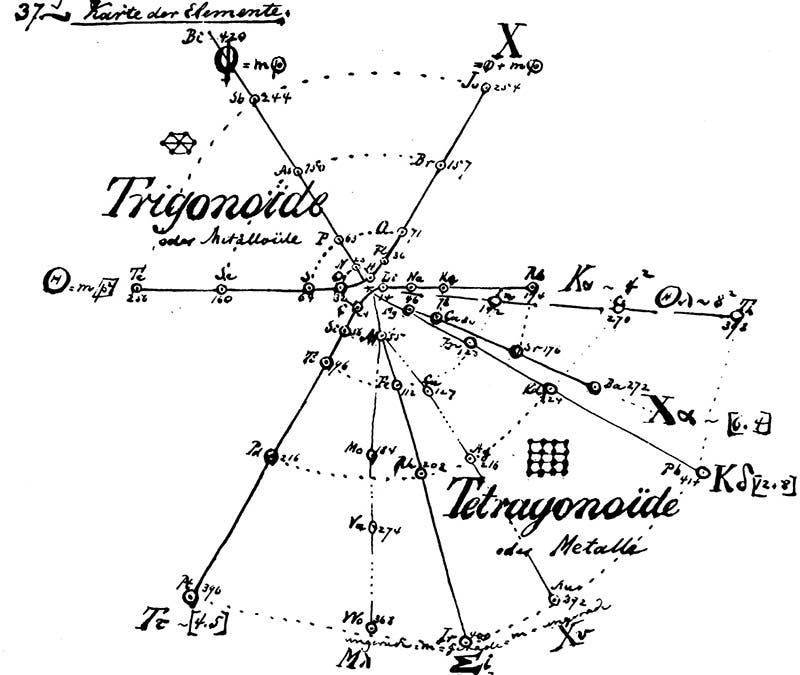
Over the next three years, Hinrichs studied the spectral lines in different elements and developed mathematical formulae relating their distribution to the sizes and weights of their constituent atoms. In 1867, he published the results of his investigations in a book entitled Programme der Atomechanik. This slim volume contained a lithographic reproduction of Hinrichs’ handwritten analysis and included a chart that divided the elements into eleven groups arranged in a spiral pattern (second image). Elements with similar chemical properties were connected by lines radiating out from the center of the chart in order of increasing atomic weight. The halogens (fluorine, chlorine, bromine, and iodine), for example, were positioned along the X-radius in the top right quadrant.
Hinrichs’ chart effectively illustrated that elements’ chemical properties tended to repeat in a predictable fashion when they were organized by atomic weight. In the end, however, his “natural classification of the elements” would be overshadowed by a new periodic table created by Russian chemist Dmitrii Mendeleev in 1869. Unlike Hinrichs, who assumed his system his system was largely complete, Mendeleev explicitly included gaps on his table and summarized the physical and chemical properties of the missing elements. When those descriptions proved accurate, other chemical classification schemes, including Hinrichs’, fell by the wayside.
Such setbacks did not prevent Hinrichs from pursuing other scientific projects. In February 1875, he witnessed what he later described as “one of the most brilliant meteors of modern times,” a fireball that was visible from Omaha to Chicago. The meteor exploded over the Amana Colonies, a religious commune approximately 25 miles (~ 40 km) west of Iowa City. Since mineralogy fell under his purview as a physical science professor, Hinrichs took it upon himself to document the event and collect any surviving meteorite fragments. He subsequently issued the definitive account of the so-called Amana meteorites and distributed samples to museum collections around the world (third image).
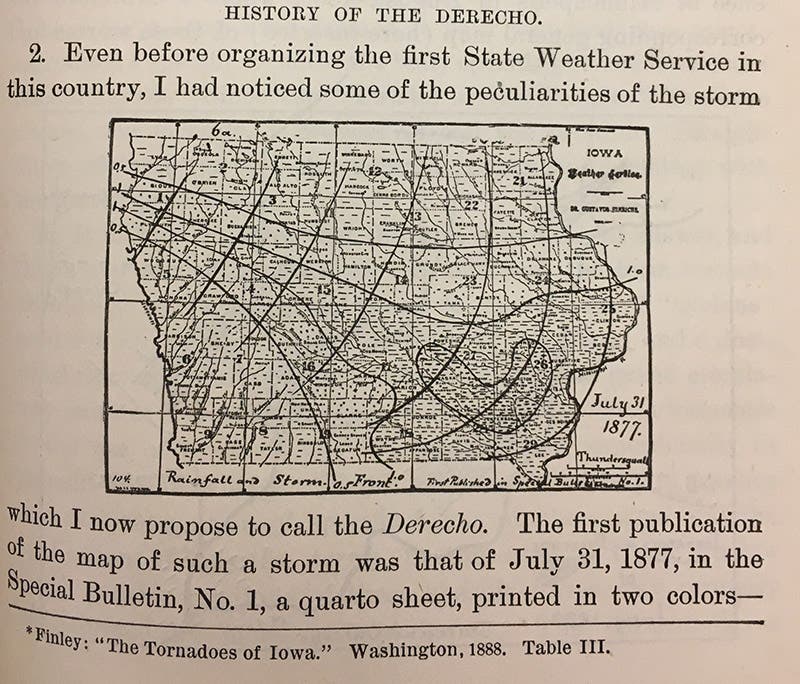
Shortly after Hinrichs finished collecting those meteorite samples, he embarked on an ambitious campaign to create the first state-wide weather service west of the Mississippi River. He transformed his Iowa City home into a meteorological station and recruited a network of volunteer observers from across the Hawkeye State. After operating independently for three years, the Iowa Weather Service secured official recognition from the state legislature in 1878. Hinrichs served as the organization’s first director and oversaw the collection and publication of weather data for over a decade. During that time, Hinrichs coined a new term to describe the lines of violent thunderstorms that occasionally swept across the plains of the Midwest. Drawing a deliberate contrast with the twisting, circular winds of the tornado, whose name was derived from the Spanish verb tornar (“to turn”), he referred to the phenomenon as a derecho: Spanish for “straight.”
Hinrichs’ neologism first appeared in print in 1888 (fourth image), but it took more than a century before it entered most meteorologists’ vocabulary. Today, however, it is not unusual to read about the devastation caused by a summertime derecho, such as the cataclysmic wave of storms that struck Iowa and Illinois in August 2020. As cleanup crews at the University of Iowa removed fallen trees and electrical lines, several journalists pointed out the cruel coincidence that the disaster that had damaged the school’s campus had first been identified by one of its most noteworthy professors.
Although Hinrichs’ research raised the University of Iowa’s scientific standing, he could be an arrogant and cantankerous colleague. He clashed regularly with other professors, as well as administrators who sought to divert funding from the sciences to the liberal arts. Eventually, his feud with the university’s president, Josiah Pickard, grew so heated that the school’s Board of Regents intervened. Hinrichs was dismissed from the faculty in 1886. He stayed on as director of the Iowa Weather Service for three years before relocating to Missouri and a new position at St. Louis University. He taught chemistry in the university’s College of Pharmacy until 1907 and remained in close contact with the scientific community even after retirement. One of his correspondents was the mining engineer Rossiter Raymond, who donated several of Hinrichs’ books to the Engineering Societies Library which, in turn, found their way into our stacks when that collection was transferred to Kansas City (fifth image).
When Gustavus Hinrichs passed away in 1923, the Iowa Academy of Science printed a brief remembrance, which praised him as “the brainiest personage that perhaps ever trod our prairie soil.” While one may take issue with that assessment, it is difficult to deny the breadth of Hinrichs’ achievements, which spanned the domains of chemistry, mineralogy, and meteorology. Perhaps more importantly, he accelerated the trend to incorporate laboratory training into undergraduate physics and chemistry classes, while simultaneously confirming that Midwestern researchers could make substantive contributions to the global scientific enterprise.
Benjamin Gross, Vice President for Research and Scholarship, Linda Hall Library. Comments or corrections are welcome; please direct to grossb@lindahall.org.