Scientist of the Day - Peter Jacob Hjelm
Peter Jacob Hjelm, a Swedish chemist, was born Oct. 2, 1746, in Sunnerbo, Småland, in southern Sweden. He attended Uppsala University and then studied and taught at a mining academy. In 1781, Hjelm was the first to isolate the metal molybdenum, which had been identified, but not isolated, by the great Swedish chemist, Carl Scheele, in 1778. There is a portrait of Hjelm on Wikipedia, but it is not well documented, so we prefer to leave him faceless for the time being.
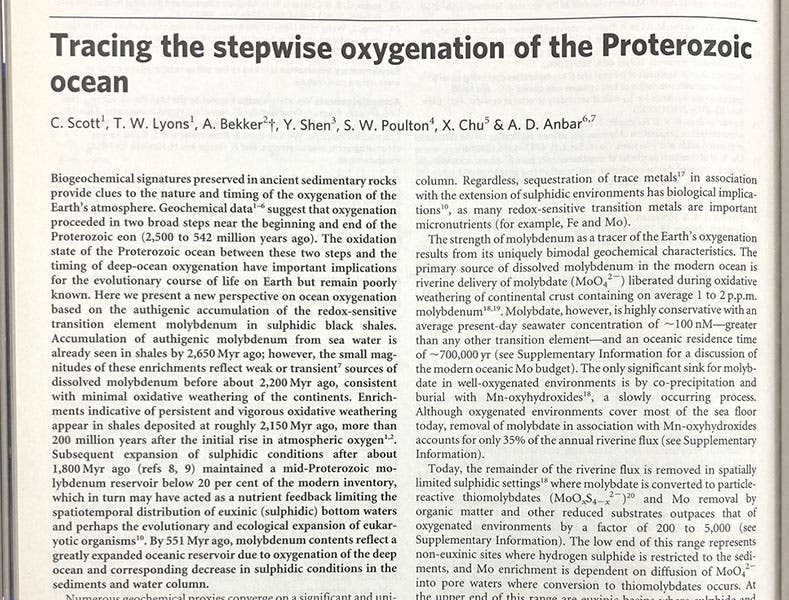
This might be just another tale of yet another Swedish element discovery – Swedes discovered over 20 elements on the periodic table (including nine in the tiny village of Ytterby outside Stockholm), mostly between 1775 and 1820 – except that molybdenum has a very special place in the history of life on earth. All living things need nitrogen, and although that gas is abundant in the atmosphere, the N2 molecule is bound so tightly that it is useless as a source for nitrogen, even though we each breathe tons of it, in and out, every day. So we depend on bacteria to extract nitrogen for us.
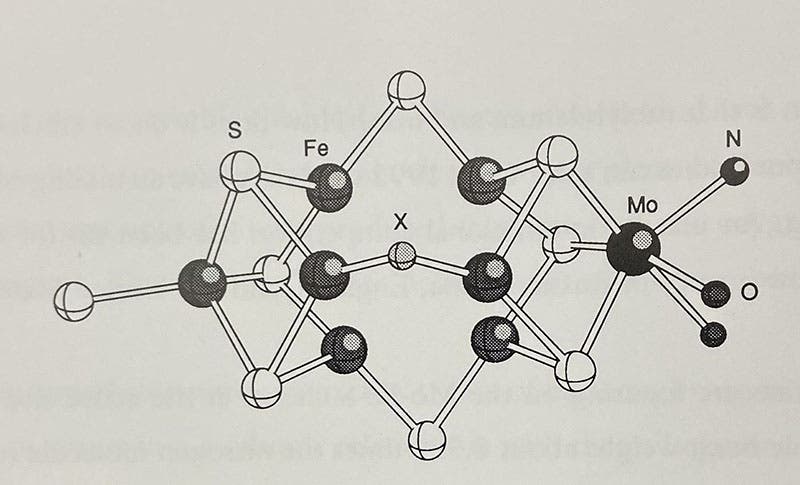
Active site (X) of a nitrogenase molecule, with 7 iron atoms (Fe) in dark grey and a single molybdenum atom (Mo) in black, diagram in Chemistry Imagined: Reflections on Science, by Roald Hoffmann, with collages by Vivian Torrence, 1993 (author’s copy)
These nitrogen-fixing bacteria have an enzyme, nitrogenase, that is an extremely large molecule, and its active site, where the nitrogen (N2) is converted to ammonia (NH3), is comprised of atoms of sulfur, iron, and yes, one single atom of molybdenum. The structure of this active site was first worked out in 1992, just in time for Roald Hoffmann to include a discussion of molybdenum and nitrogen fixation in his wonderful book, Chemistry Imagined: Reflections on Science (illustrated with collages by Vivian Torrence, 1993), where he included a ball-and-stick diagram of the active site of nitrogenase, on which X marks the location where N2 docks and NH3 emerges, and you can see 7 iron atoms in dark gray, and that single molybdenum atom, in black, without which the catalysis does not occur (second image).

Dust jacket, Chemistry Imagined: Reflections on Science, by Roald Hoffmann, with collages by Vivian Torrence, 1993 (author’s copy)
In 2008, in an article in Nature by an international team of 7 authors, it was noted that, for most of the Earth's history, all the molybdenum was locked up in rocks on the ocean floors and unavailable for nitrogen-fixing duty. It was probably not a coincidence that higher life forms (eukaryotes) did not emerge throughout this period, even though bacteria and cyanobacteria appeared 3.5 billion years ago. Not until 600 million years ago had the oxygen level in the oceans risen enough that molybdenum was released, nitrogen-fixing became possible, and the Cambrian explosion of life occurred. So the discovery of molybdenum by Hjelm in 1781 is certainly worth noticing. Maybe someday, a biochemist will discover that yttrium, terbium, erbium, or ytterbium (four of the elements discovered in tiny Ytterby) is essential for some other life process, and we will then do a post on that element's discovery.
Molybdenum is also a very attractive metal, as our photo shows (first image). Perhaps we should make it the state metal of Missouri. After all, they already share the same shorthand notation: Mo. The current state mineral of Missouri is galena, an ore of lead, after which molybdenum was named (mólybdos is Greek for lead, with which molybdenum ore was often confused). I do not believe that molybdenum is the state, provincial, or national mineral or metal of anyplace, and that should change. Such an essential metal deserves commemoration.
William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.