Scientist of the Day - Robert Boyle
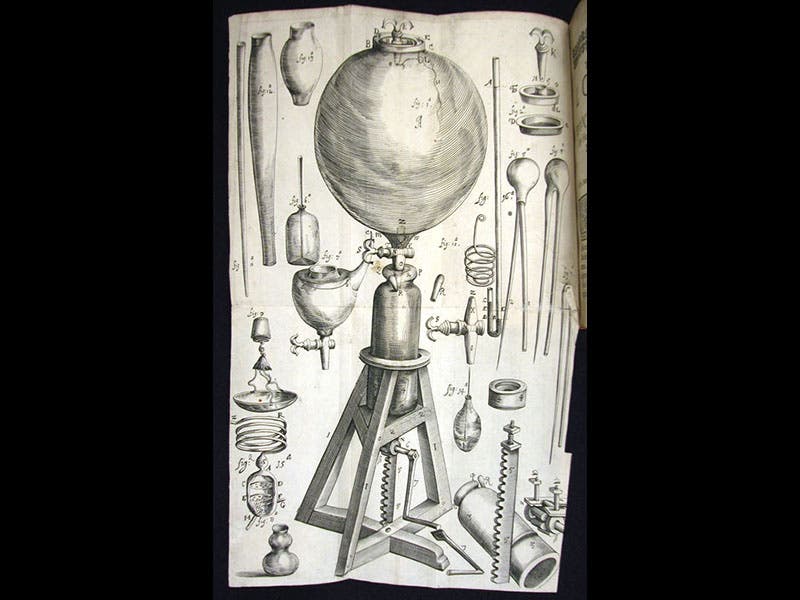



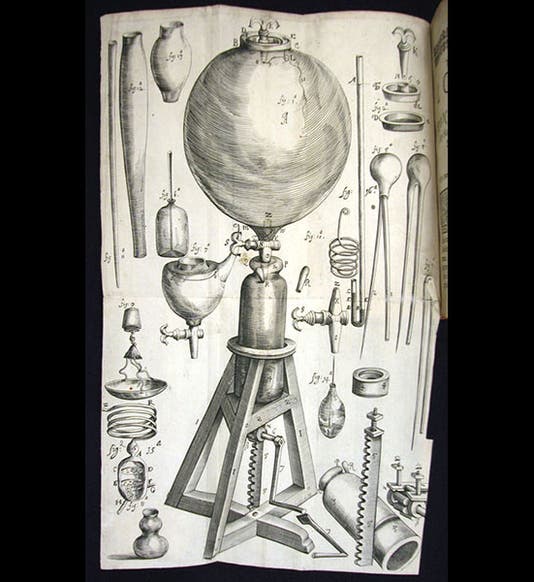
Robert Boyle, an Irish natural philosopher, was born Jan. 25, 1627. Boyle is well known for presiding over the transition from alchemy to chemistry in the 1660s and for writing the Sceptical Chymist (1661), in which he took on Paracelsian alchemists. Today we focus on his work in pneumatic chemistry and his discovery of "the spring of the air." Evangelista Torricelli had discovered, in 1644, that air has weight (or more correctly, he discovered that mercury would stay up in the tube of a barometer, which is best explained by the hypothesis that air has weight). Subsequently, Otto von Guericke learned how to take air out of a container with a vacuum pump. But he also discovered a curious thing--when you take half the air out of a closed container, the remaining half doesn't just sit at the bottom--it still seems to fill the container, even though there is less of it. Boyle began making his own air pumps in the late 1650s (or rather, he hired an assistant, Robert Hooke, to build the pumps), and as he did his own experiments, he realized that air does indeed have the curious property that Guericke suspected: it has "spring", as Boyle called it. Boyle observed that air particles behave like a handful of wool--when you squeeze the fibers, they push back. Most importantly, he discovered that the more you compress the particles, the more they push back, and their behavior is predicted by a simple mathematical law: pressure x volume is a constant. This law has been called, even since, Boyle's law.
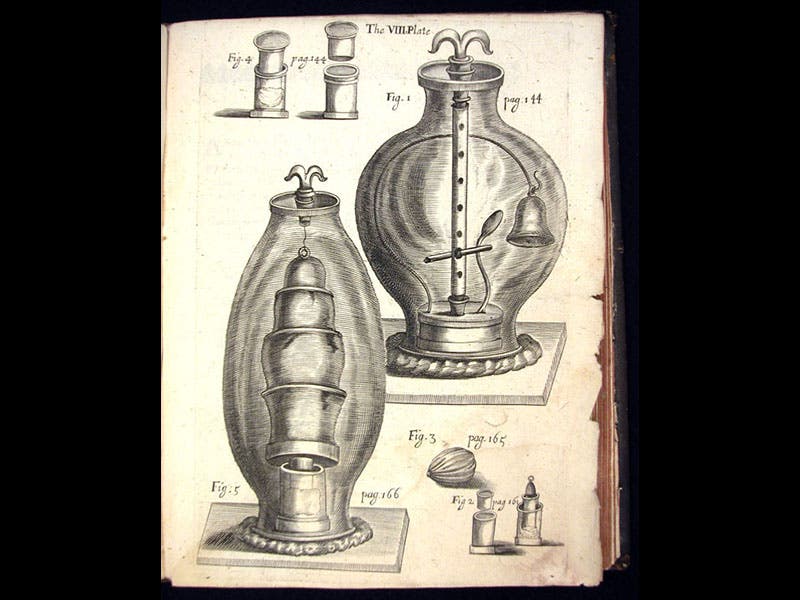
Boyle published two books on his air experiments with the same title: New experiments physico-mechanicall touching the spring of the air. The first edition of 1660, a short work, does not contain Boyle’s law, as it had not yet been discovered, but it does have an engraving of his air pump (first and fourth images); the second edition of 1662 (fifth image), which is greatly enlarged, contains the first statement of the law. We have lovely copies of both editions in our History of Science Collection. We also have Boyle’s Continuation of New Experiments (1669), which has many engravings of quite elaborate pneumatic experiments (third image).
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.