Scientist of the Day - William Ramsay
William Ramsay, a Scottish chemist, was born Oct. 2, 1852. In the early 1890s, it was discovered by Lord Rayleigh that nitrogen produced in the laboratory is slightly lighter than atmospheric nitrogen, suggesting that there was some other gas in the atmosphere as yet undetected. Ramsay heard Rayleigh give a talk on the problem and offered to look for the mystery gas. Ramsay was a master of analytic chemical techniques that could be used to scrub gases such as nitrogen, oxygen, and carbon dioxide out of the atmosphere to see what was left, and it 1894, he succeeded in isolating an atmospheric gas that had an atomic number of 18 or 19, a unique spectrum, and did not react with any other elements. It was truly an inert gas. He and Rayleigh named it "argon," the "lazy" gas, and announced its existence to the Royal Society of London on Jan. 31, 1895. Their initial presentation was published in the Proceedings of the Society, with a lengthier paper appearing soon after in the Philosophical Transactions (second image). Both papers were followed immediately in the journal issues by papers by William Crookes defining the spectrum of argon.
The main problem raised by argon was that it did not fit easily into Mendeleev's periodic table, which by 1894 had already become a backbone of chemistry. Thus many chemists, including Mendeleev himself, initially rejected argon as an element, suggesting it might be some form of tri-atomic nitrogen. But just two months after his argon paper, Ramsay discovered terrestrial helium, another inert gas (helium had actually been spectroscopically discovered in the Sun by Norman Lockyer and Jules Janssen back in 1868, but Ramsay was the first to isolate it on Earth.) He announced the discovery in Chemical News in March of 1895 (third image)
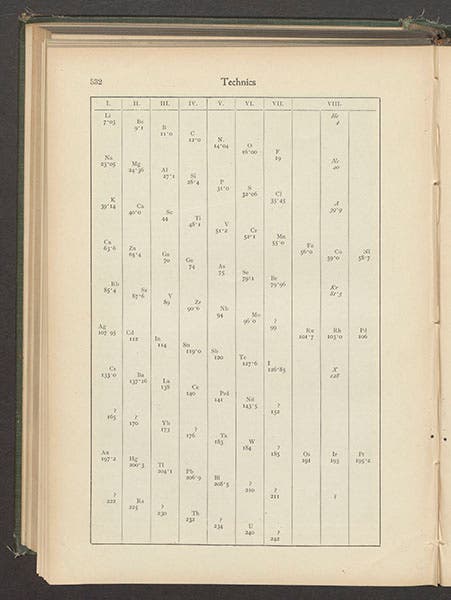
And then, in 1898, working with Morris Travers, Ramsay discovered three more inert gases in quick succession: krypton, neon, and xenon. The solution to the periodic table problem was now obvious – there had been a whole column missing, at the far right, nwith helium at the top, and the rest of what we now call the noble gases directly below. Ramsay published an article on the periodic table in 1904 in the short-lived journal Technics, where he included an eighth column for his five new gases (fourth image)
In a 1908 caricature of Ramsay in Vanity Fair, he points to the new column of the periodic table (fifth image). For discovering the noble gases, Ramsay received the fourth Nobel Prize in Chemistry in 1904.
In 1995, Peter W. Atkins published an engaging book, The Periodic Kingdom, in which he imagined the periodic table as a continent, much like North America, with the reactive alkalis on the West Coast, the metallic desert in the Midwest, and the materials of life, like carbon and oxygen, in the Northeast. Using this metaphor, we might then say that Ramsay discovered the entire Atlantic Seaboard, which wasn’t even suspected to exist before he set out.
Four years ago, our own Ben Gross wrote a Science Time Capsule about Ramsay for WHYY in Philadelphia, which is still online and still well worth reading. Dr. Gross also found Ramsay’s article in Technics, with the periodic table that may be the first in print to incorporate the noble gases.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.