Scientist of the Day - Harold Urey
Harold Clayton Urey, an American chemist, was born Apr. 29, 1893. In 1931, working at Columbia University in New York, Urey discovered deuterium, an isotope of hydrogen. It was not clear at first just what deuterium is, since the neutron would not be discovered until the next year, but it soon turned out that deuterium has a nucleus consisting of a proton and a neutron. Since deuterium behaves chemically just like hydrogen, it can combine with oxygen to form water – “heavy water", that would later find a use in nuclear reactors. Urey was the first to make heavy water. For his discovery of deuterium, Urey received the Nobel Prize in 1934, a remarkably quick turn-around for a Nobel award. During World War II, Urey was in charge of developing methods to separate the isotope Uranium-235 from the more abundant U-238, ultimately settling on a method known as gaseous diffusion, which proved successful.
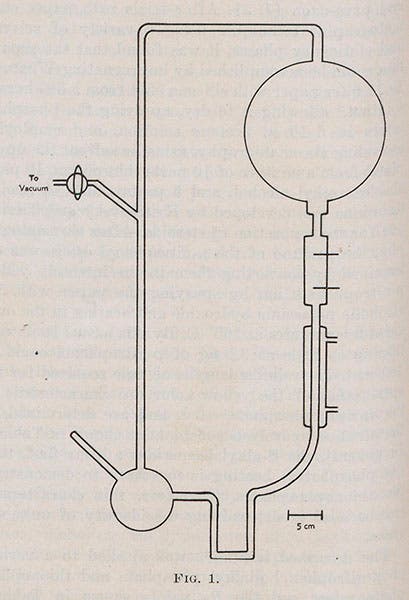
After the war, Urey moved to the University of Chicago, and for the remainder of this notice, we would like to discuss a discovery that Urey himself did not make, but for which he was absolutely essential. Urey gave a lecture in 1951 in which he decried the lack of effort being made to test the idea that a “primordial soup” on the early earth had given rise to life, an idea that had been advanced by J.B.S. Haldane and Alexandr Oparin in the 1920s. Urey suggested that the earth's primitive atmosphere probably consisted of a mixture of carbon dioxide, water vapor, ammonia, and methane, and that experiments could be done recreating the conditions of the early earth. Listening to that lecture was a young graduate student, Stanley Miller, and in 1952, Miller asked permission to do such an experiment under Urey’s supervision for his doctoral thesis. Urey at first refused, since in spite of his pep talk, he was not at all sure that the proposed experiment would succeed. But Miller was persuasive, Urey agreed to mentor him, and Miller set up a laboratory experiment in which he placed the four proposed primordial gases in a flask, subjected them to electric sparks, circulated the gases through a condenser, and found that he was able to make several amino acids, organic molecules that are constituents of proteins. The diagram shows how simple the experiment was (third image).
Miller sent his paper off to Science, the premier scientific journal in the U.S., in December of 1952, and when he did not hear back for quite a while, he mentioned this to Urey. Urey called up the editor and demanded the paper back, saying they would find a more responsive journal for it; the editor relented on the spot and guaranteed publication within months. You do not want to try this unless you have a Nobel Prize in your pocket – editors of science journals ordinarily do not take guff from anyone, except the laurelled few, like Urey. The best part of the whole story is that Urey refused to have his name added as coauthor (which was customary for a PhD supervisor), saying that if his name were on the paper, no one would give Miller any credit. As it was, Miller got a great deal of credit, and he became as famous as any graduate student is likely to become, especially when the popular press found out that Miller had made “life in a test tube” (which of course he hadn’t).
In spite of Urey’s disavowals, Miller’s experiment is often referred to as the “Miller-Urey experiment” (that happens to be the title of the Wikipedia article on the subject), and sometimes as the Urey-Miller experiment, which Urey really disliked.
Our other images show Urey at his desk; Urey in 1942 with members of the S-1 Uranium Committee (second image; Urey is at far left); Miller’s complete two-page paper of 1953 in Science (fourth image); and a detail of the opening of Miller’s paper, where he credits Urey (fifth image). Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.






![“Aurora Borealis,” hand-colored wood engraving by Josiah Wood Whymper, [Natural Phenomena], plate 2, 1846 (Linda Hall Library)](https://assets-us-01.kc-usercontent.com:443/9dd25524-761a-000d-d79f-86a5086d4774/0245ffcb-b70c-477c-8792-0a73ebd54eb2/Whymper%2011.jpg?w=210&h=210&auto=format&fit=crop)