Scientist of the Day - John Frederic Daniell
John Frederic Daniell, an English chemist, was born Mar. 12, 1790. Daniell is best known for devising a new kind of battery in 1836. The first electric battery had been invented by Alessandro Volta in 1800, but the Voltaic pile, as it was called, had several drawbacks – it was not particularly portable, and it used sulfuric acid as a solution, which means it released inflammable hydrogen gas. The Daniell cell, as it was almost immediately called, used two solutions, zinc sulfate and copper sulfate, rather than sulfuric acid, and hence gave off no hydrogen bubbles.
The original Daniell cell had the zinc sulfate and the copper sulfate in separate but adjacent jars, connected by a "salt bridge", a kind of pipe that let the two sulfate solutions circulate, but soon it was realized that if one put a ceramic jar inside a copper container, and the zinc solution inside the ceramic jar, the ceramic would effectively separate the two solutions, but would still allow the sulfate ions to circulate through the porous pottery wall. This is the form the Daniell cell would have for the next 60 years. Since it was portable and safe, the Daniell cell was used to power the communications on all the attempts to lay an Atlantic cable – the temporarily successful one of 1858, and the more permanently successful effort of 1866. When they came to first defining international electrical standards in the 1860s, the output of the Daniell cell was used to determine the unit of electrical potential, which was named the volt. Revisions to the definition of a volt have left the Daniell cell with an output of about 1.07 volts. We see here a tray of 6 Daniell cells (the original 6-volt battery) as displayed at the Smithsonian Institution.
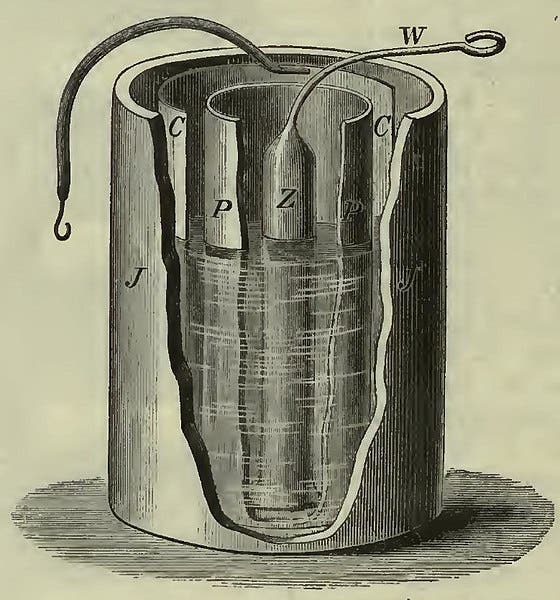
To meteorologists, Daniell is better known for inventing the wet-bulb hygrometer. This was an earlier achievement – Daniell built his first one around 1820, then announced it in his 1823 book, Meteorological Essays and Observations. The frontispiece of the book shows a diagram of the new hygrometer (third image). Evaporation from the wet bulb lowers the temperature on the enclosed thermometer, but the amount of lowering depends on how much moisture the atmosphere already holds, i.e., its humidity.
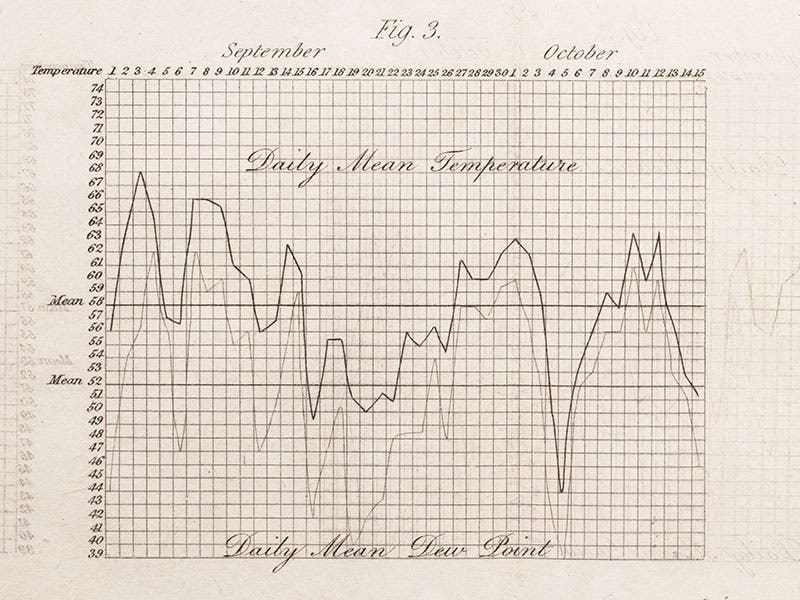
Daniell devised his hygrometer to measure the dew point of the air, an important factor in weather prediction. He used it to make daily measurements of dew point versus temperature, the results of which he presented on graphs at the end of his book, such as the one we show here (fourth image).
Daniell's book, held by our Library, was also part of the Library of HMS Beagle on Charles Darwin's voyage of 1831-36, but it was probably not selected by Darwin, but rather by Robert Fitzroy, who was passionate about nautical meteorology. Whether the Beagle carried a Daniell hygrometer, we do not know. But there are many of his wet-bulb hygrometers that still survive, on display in a variety of museums, including this one in the Science Museum, London (fifth image).
Daniell died of a stroke at a rather young age, the day after his 55th birthday. There is a handsome oil portrait of him at King’s College, London, but we prefer this daguerreotype of Daniell with the great Michael Faraday, which is held by the Royal Institution (sixth image).
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.